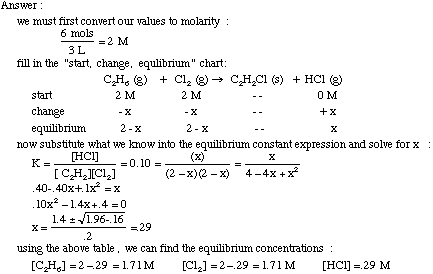Learn how to calculate an equilibrium constant Kp. | Chemistry help, Teaching chemistry, High school chemistry
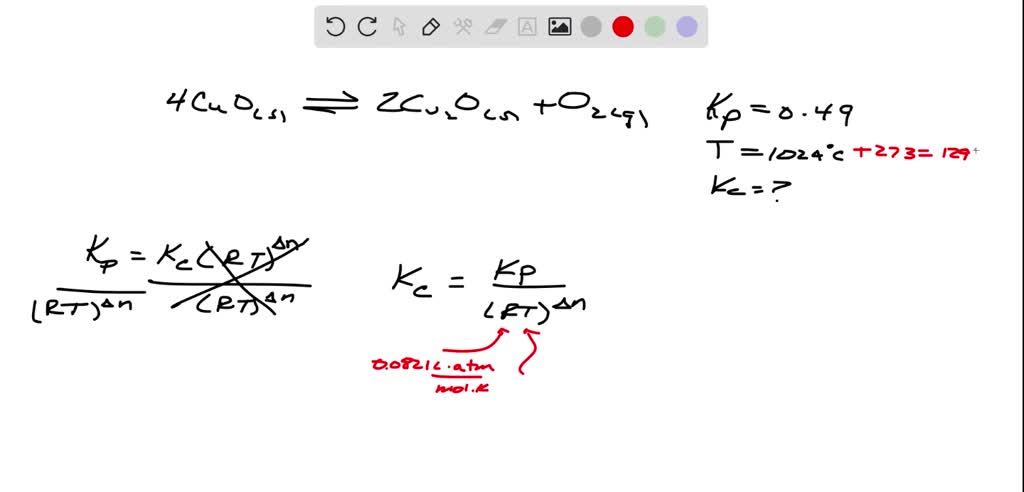
SOLVED: Kp for the reaction: 4CuO (s) <—-> 2Cu2O (s) + O2 (g), is 0.49 at 1024 ° C. Calculate Kc a this temperature.
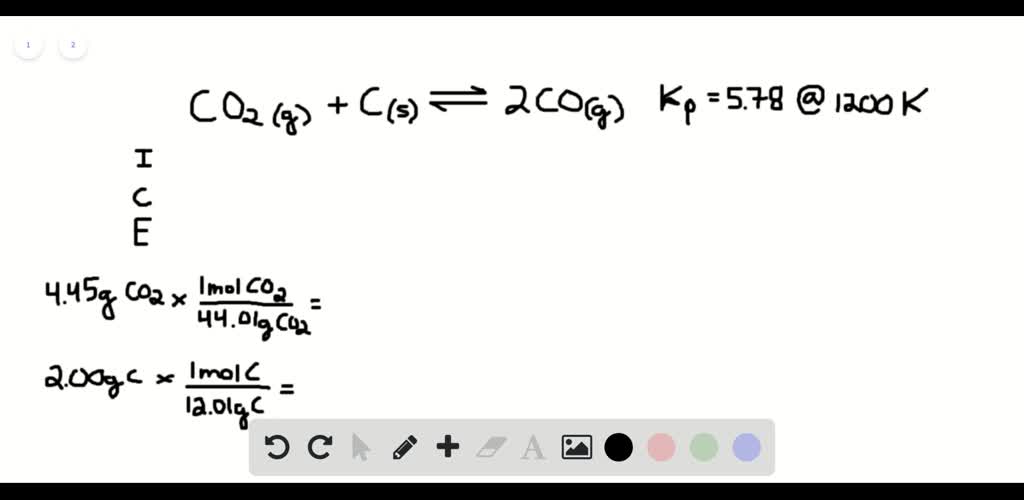
SOLVED:The reaction CO2(g) + C(s) 2 CO(g) has Kp = 5.78 at 1200 K. a. Calculate the total pressure at equilibrium when 4.45 g of CO2 is introduced into a 10.0-L container